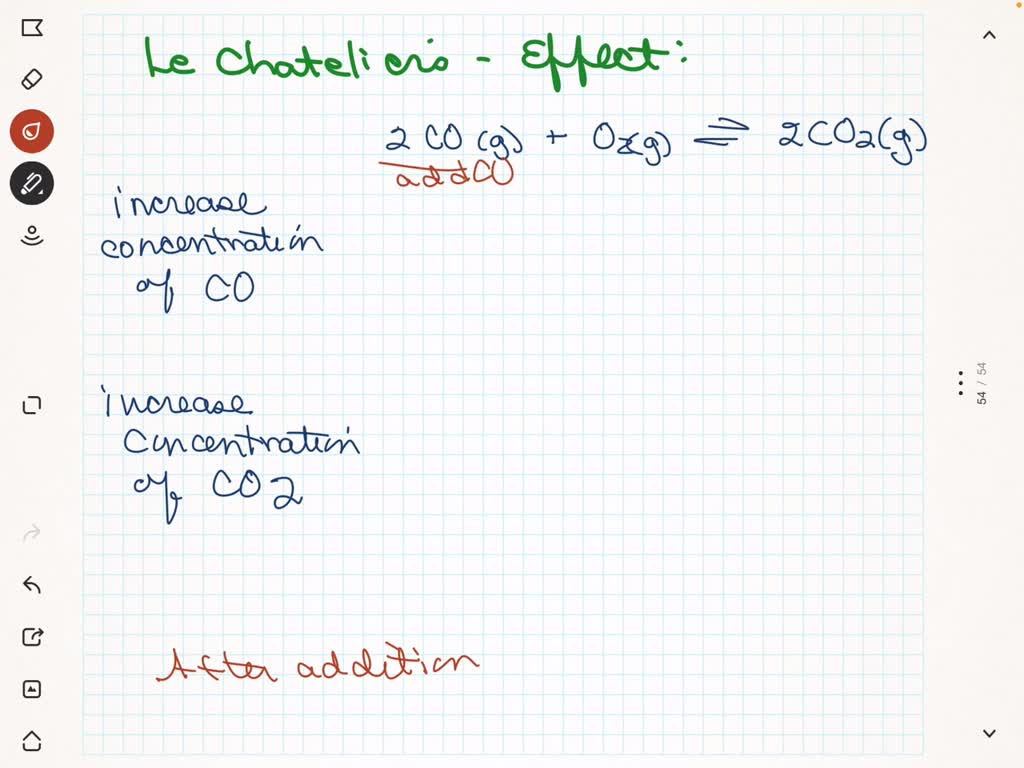
Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after reactant or product is added. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leaving it blank means there is no change in the concentration. 2CO(g) + O2(g) = 2CO2(g)
3 The concentrations of each major carbonate species vs pH for Brine #1… | Download Scientific Diagram
See Answer. Question: For each event stated, indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leavin it blank means there is no change in the concentration. increasing the

Source Image: sciencenews.org
Download Image
Calculate the equilibrium concentration for each species from the initial concentrations and the changes. [H 2] = [Br 2] = 0.010 – x = 0.010 – 0.008 = 0.002 M for each [HBr] = 2x = 2(0.008) = 0.016 M. Check your answer by substituting the equilibrium concentrations into the equilibrium expression and see if the result is the same as the equilibrium constant.

Source Image: healthline.com
Download Image
Exploring the Color of Tea – Yunomi.life . Consider an equilibrium mixture consisting of H2O (g), CO (g). H2 (g), and CO2 (g) reacting in a closed vessel according to the equation H2O (g)+CO (g)H2 (g)+CO2 (g)a. You add more H2O to the flask. How does the new equilibrium concentration of each chemical compare to its origin al equilibrium concentration after equilibrium is re-established?

Source Image: nbcnews.com
Download Image
Indicate How The Concentration Of Each Species
. Consider an equilibrium mixture consisting of H2O (g), CO (g). H2 (g), and CO2 (g) reacting in a closed vessel according to the equation H2O (g)+CO (g)H2 (g)+CO2 (g)a. You add more H2O to the flask. How does the new equilibrium concentration of each chemical compare to its origin al equilibrium concentration after equilibrium is re-established? Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after reactant or product is added. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leaving it blank means there is no change in the concentration. 2C0 (g) + 0, (g) = 2C0, (g
Many common household cleaning products can kill the coronavirus if you use them properly
Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after reactant or product is added. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leaving it blank means there is no change in the concentration. 200 (8) +0, () 2CO (9 Do more plants invite more insects? Study explores the question

Source Image: researchmatters.in
Download Image
SOLVED: Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after a decrease in pressure or volume. If there is no change in the concentration Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after reactant or product is added. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leaving it blank means there is no change in the concentration. 200 (8) +0, () 2CO (9

Source Image: numerade.com
Download Image
3 The concentrations of each major carbonate species vs pH for Brine #1… | Download Scientific Diagram Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after reactant or product is added. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leaving it blank means there is no change in the concentration. 2CO(g) + O2(g) = 2CO2(g)

Source Image: researchgate.net
Download Image
Exploring the Color of Tea – Yunomi.life Calculate the equilibrium concentration for each species from the initial concentrations and the changes. [H 2] = [Br 2] = 0.010 – x = 0.010 – 0.008 = 0.002 M for each [HBr] = 2x = 2(0.008) = 0.016 M. Check your answer by substituting the equilibrium concentrations into the equilibrium expression and see if the result is the same as the equilibrium constant.

Source Image: yunomi.life
Download Image
SOLVED: Indicate how the concentration of each species in the chemical equation will change when a product is added. An up arrow indicates an increase in concentration, down arrow indicates a decrease 100%. Transcribed Image Text: Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after increasing the pressure. N2 (g) + 3 H, (g) 2 NH; (g) The concentration of N, will stay the same. increase. decrease. The concentration of H, will stay the same. increase. decrease.
Source Image: numerade.com
Download Image
French Perfume – the powerful fragrance . Consider an equilibrium mixture consisting of H2O (g), CO (g). H2 (g), and CO2 (g) reacting in a closed vessel according to the equation H2O (g)+CO (g)H2 (g)+CO2 (g)a. You add more H2O to the flask. How does the new equilibrium concentration of each chemical compare to its origin al equilibrium concentration after equilibrium is re-established?

Source Image: vovworld.vn
Download Image
SOLVED: Indicate how the concentration of each species in the chemical equation will change when a product is added. An up arrow indicates an increase in concentration, down arrow indicates a decrease Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after reactant or product is added. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leaving it blank means there is no change in the concentration. 2C0 (g) + 0, (g) = 2C0, (g

Source Image: numerade.com
Download Image
SOLVED: Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after a decrease in pressure or volume. If there is no change in the concentration
SOLVED: Indicate how the concentration of each species in the chemical equation will change when a product is added. An up arrow indicates an increase in concentration, down arrow indicates a decrease See Answer. Question: For each event stated, indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leavin it blank means there is no change in the concentration. increasing the
Exploring the Color of Tea – Yunomi.life French Perfume – the powerful fragrance 100%. Transcribed Image Text: Indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after increasing the pressure. N2 (g) + 3 H, (g) 2 NH; (g) The concentration of N, will stay the same. increase. decrease. The concentration of H, will stay the same. increase. decrease.